Your Percent ionic character formula images are ready in this website. Percent ionic character formula are a topic that is being searched for and liked by netizens today. You can Get the Percent ionic character formula files here. Find and Download all free images.
If you’re looking for percent ionic character formula images information related to the percent ionic character formula keyword, you have visit the right site. Our website always gives you suggestions for seeking the maximum quality video and picture content, please kindly surf and find more informative video content and images that fit your interests.
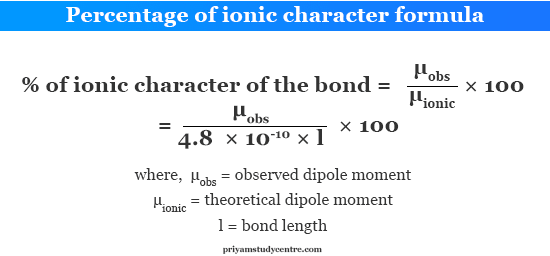
Percent Ionic Character Formula. Eb Electronegativity of atom B. In esu units esu means electrostatic units the fundamental charge is given as e 480320440 1 x 10 -10 esu. Percentage of ionic character. The formula is given below.

Dipole moment of KCl is 3336 10 29 coulomb metre which indicates that it is highly polar molecule. The percent ionic character is then just As things stand this is really rather awkward. For the calculation of percentage ionic character remember the conversion units ie 1 D 3335 10 30 c m. If we remember that 1Å 10 -8 cm then 1D 10 -18 esucm 10 -10 esuÅ. Percentage of ionic character. But the μ of AB is neither zero nor μ ionic.
Ea - Eb The subtraction will give a positive value always since modulus modulus sign is used.
Percentage ionic character of HCl If we consider HCl as a purely ionic compound the charge on hydrogen and chlorine 48 10 -10 esu and bond length 127 10 -8 cm. Percentage of ionic character. Dipole moment of KCl is 3336 10 29 coulomb metre which indicates that it is highly polar molecule. How to determine the percent ionic character. But the μ of AB is neither zero nor μ ionic. Ea - Eb The subtraction will give a positive value always since modulus modulus sign is used.
 Source: priyamstudycentre.com
Source: priyamstudycentre.com
Therefore to determine the percent ionic character of hydrogen fluoride the measured value of 118 D is divided by 441 D which gives a percent ionic character of 41. 16 Ea - Eb 35 Ea - Eb2 Where Ea Electronegativity of atom A. For calculating the percentage of ionic character in covalent bond we simply need to use a formula. Percentage ionic character of HCl If we consider HCl as a purely ionic compound the charge on hydrogen and chlorine 48 10 -10 esu and bond length 127 10 -8 cm. Percent ionic character 1 e Δ χ 2 2 100.
 Source: youtube.com
Source: youtube.com
Percent ionic character μ observed μ calculated 100. Higher magnitude of this value represents that the bonding is more ionic in nature is calculated using Percent Ionic Character100 1-exp -025 Electronegativity of element A-Electronegativity of element B2. Percent_ionic_character 100 1-exp-025 Electronegativity of element A-Electronegativity of element B2 ionic character 100 1-exp-025 XA-XB2 This formula uses 1 Constants 1 Functions 2 Variables. Percentage ionic character of HCl If we consider HCl as a purely ionic compound the charge on hydrogen and chlorine 48 10 -10 esu and bond length 127 10 -8 cm. In esu units esu means electrostatic units the fundamental charge is given as e 480320440 1 x 10 -10 esu.
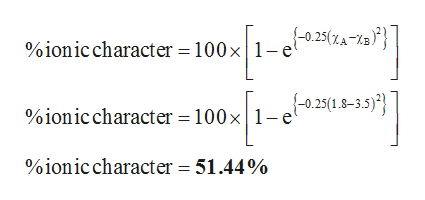 Source: bartleby.com
Source: bartleby.com
How to determine the percent ionic character. In esu units esu means electrostatic units the fundamental charge is given as e 480320440 1 x 10 -10 esu. What is the percent ionic character of Mg3N2. Calculate the percent ionic character of HF HCl HBr HI and CsF and compare the results with those in Table 37. Eb Electronegativity of atom B.
 Source: youtube.com
Source: youtube.com
But the μ of AB is neither zero nor μ ionic. Percent ionic character 1 e Δ χ 2 2 100. Percent ionic character μ observed μ calculated 100. The ionic character is nearly 4325 so the covalent character is 100 4325 5675. Ea - Eb The subtraction will give a positive value always since modulus modulus sign is used.

Eb Electronegativity of atom B. The percent ionic character is then just As things stand this is really rather awkward. If we remember that 1Å 10 -8 cm then 1D 10 -18 esucm 10 -10 esuÅ. Reactivity order of Pyrrole Furan and Thiophene towards Electrophilic substitution. What is the percent ionic character of Mg3N2.
 Source: toppr.com
Source: toppr.com
For calculating the percentage of ionic character in covalent bond we simply need to use a formula. Materials science and engineering tutorial. The procedure is put in your electronegativities. If we remember that 1Å 10 -8 cm then 1D 10 -18 esucm 10 -10 esuÅ. Percentage of ionic character 16 Ea - Eb 35 Ea - Eb2 Where Ea Electronegativity of atom A.

The percent ionic character of a bond can be approximated by the formula is the magnitude of the difference in the electronegativities of the atoms see Fig. Reactivity order of Pyrrole Furan and Thiophene towards Electrophilic substitution. Dipole moment of KCl is 3336 10 29 coulomb metre which indicates that it is highly polar molecule. A covalent bond with equal sharing of the charge density has 0 ionic character and a perfect ionic bond would of course have 100 ionic character. One method of estimating the percent ionic character is to set it equal to the ratio of the observed dipole moment to the value of eR all multiplied by 100.

The formula is given below. Percent_ionic_character 100 1-exp-025 Electronegativity of element A-Electronegativity of element B2 ionic character 100 1-exp-025 XA-XB2 This formula uses 1 Constants 1 Functions 2 Variables. Percent ionic character μ observed μ calculated 100. Therefore to determine the percent ionic character of hydrogen fluoride the measured value of 118 D is divided by 441 D which gives a percent ionic character of 41. Higher magnitude of this value represents that the bonding is more ionic in nature is calculated using Percent Ionic Character100 1-exp -025 Electronegativity of element A-Electronegativity of element B2.

Oleum and its percentage labbeling 1 Oleum can be represented by the formula ySO 3 H 2 O where y is the total molar sulphur trioxide content the value of y can be va. Dipole moment of KCl is 3336 10 29 coulomb metre which indicates that it is highly polar molecule. Eb Electronegativity of atom B. Ionic character 1823984 100 4568 When electronegativity difference between two atoms is 21 there is 50 ionic character in the bond. C Now percentage ionic character exp v a l u e t h e o r e t i c a l v a l u e 100 103 D 61169 D 100 1683 Note.

The formula is given below. Therefore to determine the percent ionic character of hydrogen fluoride the measured value of 118 D is divided by 441 D which gives a percent ionic character of 41. The percent ionic character is then just As things stand this is really rather awkward. Higher magnitude of this value represents that the bonding is more ionic in nature is calculated using Percent Ionic Character100 1-exp -025 Electronegativity of element A-Electronegativity of element B2. Ea - Eb The subtraction will give a positive value always since modulus modulus sign is used.
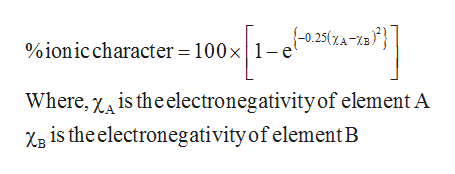 Source: bartleby.com
Source: bartleby.com
6 rows Therefore to determine the percent ionic character of hydrogen fluoride measure value of 1. The percent ionic character Observed dipole momentCalculated dipole moment assuming 100 ionic bond 100. In the alkaline earth metal group down the group as the size increases the ability to lose electrons ie. Percent_ionic_character 100 1-exp-025 Electronegativity of element A-Electronegativity of element B2 ionic character 100 1-exp-025 XA-XB2 This formula uses 1 Constants 1 Functions 2 Variables. Percent ionic character μ observed μ calculated 100.
 Source: study.com
Source: study.com
The percent ionic character of a bond can be approximated by the formula is the magnitude of the difference in the electronegativities of the atoms see Fig. Higher magnitude of this value represents that the bonding is more ionic in nature is calculated using Percent Ionic Character100 1-exp -025 Electronegativity of element A-Electronegativity of element B2. Materials science and engineering tutorial. Dipole moment of KCl is 3336 10 29 coulomb metre which indicates that it is highly polar molecule. Since the percent ionic character of hydrogen fluoride is less than 50 it is a polar covalent bond.

The formula is given below. The percent ionic character Observed dipole momentCalculated dipole moment assuming 100 ionic bond 100. Yet the percent ionic character of Mg-N bonds is 52 48 covalent character. 16 Ea - Eb 35 Ea - Eb2 Where Ea Electronegativity of atom A. Percent ionic character 1 e Δ χ 2 2 100.

Percentage of ionic character 16 Ea - Eb 35 Ea - Eb2 Where Ea Electronegativity of atom A. Eb Electronegativity of atom B. Percent_ionic_character 100 1-exp-025 Electronegativity of element A-Electronegativity of element B2 ionic character 100 1-exp-025 XA-XB2 This formula uses 1 Constants 1 Functions 2 Variables. Percent ionic character μ observed μ calculated 100. 16 Ea - Eb 35 Ea - Eb2 Where Ea Electronegativity of atom A.
 Source: youtube.com
Source: youtube.com
Percent ionic character 1 e Δ χ 2 2 100. Percent_ionic_character 100 1-exp-025 Electronegativity of element A-Electronegativity of element B2 ionic character 100 1-exp-025 XA-XB2 This formula uses 1 Constants 1 Functions 2 Variables. Worked example problem solution for percent ionic character calculation. Materials science and engineering tutorial. Why does ionic character increase down group.
 Source: youtube.com
Source: youtube.com
The ionic character is nearly 4325 so the covalent character is 100 4325 5675. Percentage of ionic character 16 Ea - Eb 35 Ea - Eb2 Where Ea Electronegativity of atom A. Higher magnitude of this value represents that the bonding is more ionic in nature is calculated using Percent Ionic Character100 1-exp -025 Electronegativity of element A-Electronegativity of element B2. Percent_ionic_character 100 1-exp-025 Electronegativity of element A-Electronegativity of element B2 ionic character 100 1-exp-025 XA-XB2 This formula uses 1 Constants 1 Functions 2 Variables. 6 rows Therefore to determine the percent ionic character of hydrogen fluoride measure value of 1.
 Source: clutchprep.com
Source: clutchprep.com
A covalent bond with equal sharing of the charge density has 0 ionic character and a perfect ionic bond would of course have 100 ionic character. Using the difference of ionic electronegativity expresseddesignated as XA and XB. But the μ of AB is neither zero nor μ ionic. Percentage of ionic character. Dipole moment of KCl is 3336 10 29 coulomb metre which indicates that it is highly polar molecule.

Using the difference of ionic electronegativity expresseddesignated as XA and XB. 16 Ea - Eb 35 Ea - Eb2 Where Ea Electronegativity of atom A. The formula is given below. Percentage ionic character formula In that case the μ of AB would be μ ionic e l 48 10 -18 esu cm. How to determine the percent ionic character.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title percent ionic character formula by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






